
Schedule a Presentation With Us!
- PapaCarie for Caries Removal
- PerioMonitor Saliva Test
- Woodpecker Laser Smart Blue
- Woodpecker Endodontic

We use cookies!
We use cookies to improve your website experience and analyze traffic. These cookies are necessary for the site to function properly and help us understand how you use it. The information collected remains anonymous and is used for analysis purposes only. By continuing to browse our site, you accept the use of these cookies. If you do not want your data to be used in this way, please click decline. You can read our document on the potection of personal information (Law 25) here
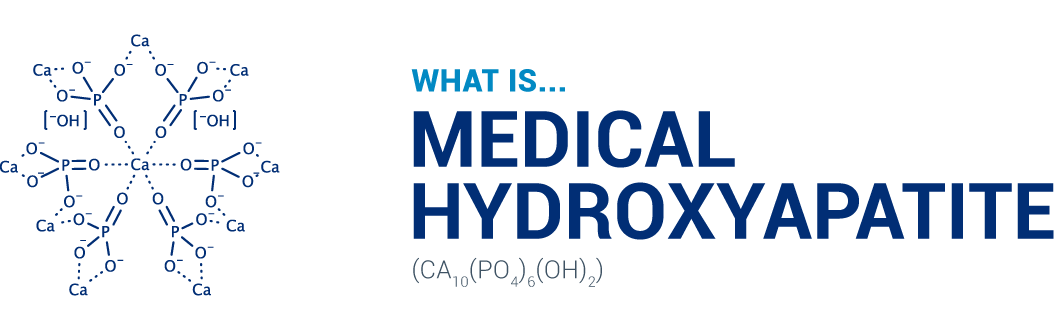

The main substance of our teeth is hydroxyapatite (97 % of enamel and 70 % of dentin), a calcium phosphate compound. This ingredient is safe and highly biocompatible. Nano medical hydroxyapatite (<mHAP>) particles penetrate below the surface of the enamel, providing replacement calcium and phosphate ions to areas from which minerals have dissolved, thereby remineralizing the demineralized enamel and restoring its integrity and translucent gloss.
• Causes remineralization comparable to a fluoride toothpaste and inhibits caries development, thus suggesting that a nano medical hydroxyapatite toothpaste can be an effective alternative to fluoride toothpastes.1
• Remineralizes subsurface demineralized areas of tooth enamel.2
• Remineralizes tooth enamel more effectively than saliva and just as effectively as fluoride compounds.
Note : fluoride promotes remineralization by saliva, where as nano medical hydroxyapatite itself
remineralizes the teeth.3
• Fills and repairs minute surface defects on tooth enamel, restoring enamel to its original smoothness.4
• Adheres to and helps remove bacteria and biofilm, thereby protecting against tooth decay.5
• Removes white spots and incipient caries and restores enamel almost to its original form.6
• Protects against biofilm attachment and stains by reducing the crevices that harbor them.7
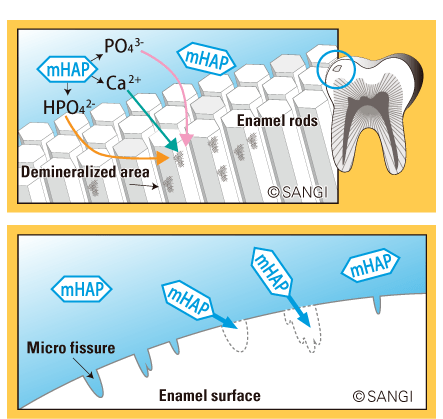

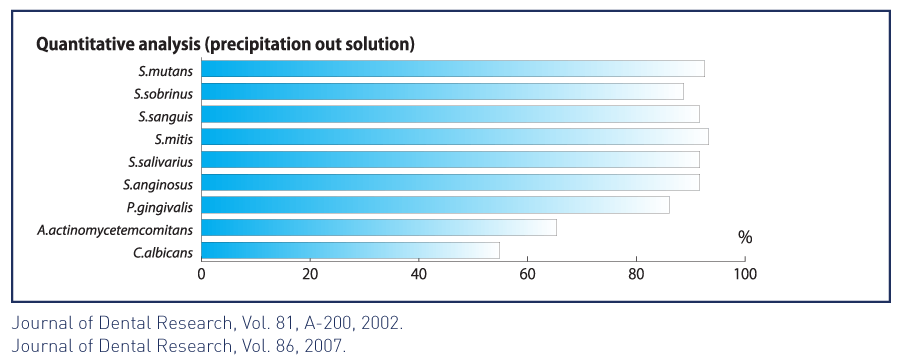
• Absorbs periodontal and opportunistic pathogens such as p. gingivalis and candida, helping reduce the risk of oral soft tissue infection.8
• Shows superior bacterial absorption compared to other forms of hydroxyapatite.9
• Shows strong selectivity for cariogenic streptococcus mutans.10
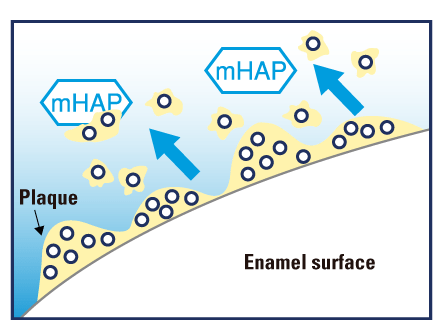
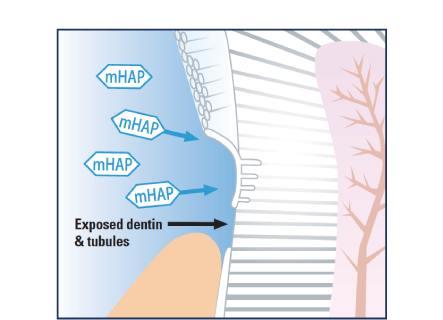
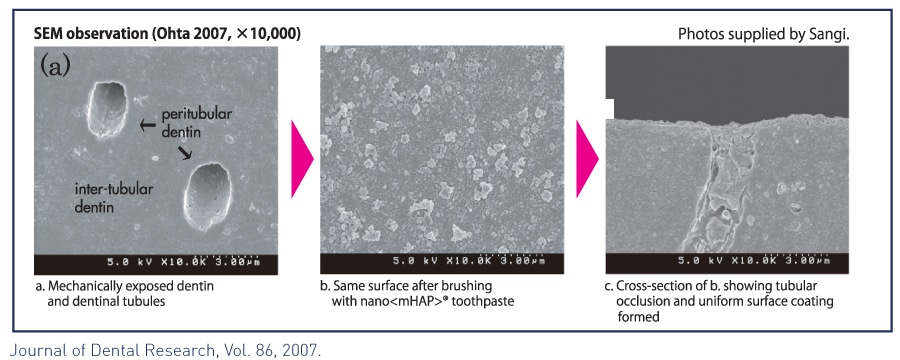
Medical Hydroxyapatite nanoparticles occlude and offer a protective coating beyond comparison over exposed dentinal tubules, providing quick and effective relief from refractory tooth hypersensitivity.
Coats and fills exposed dentin and dentinal tubules protecting against dentinal hypersensitivity.11
1970:
First synthetic form of hydroxyapatite created by NASA to help restore teeth and bone loss by astronauts following gravity-free environment missions.
1978:
Sangi Co., Ltd. acquires the patent from NASA and conceives the idea of an enamel-restorative toothpaste using the same substance as the tooth structure (hydroxyapatite).
1980:
Sangi launches the world’s first enamel-restorative toothpaste in Japan.
1985:
Studies from Tokyo Medical and Dental University (1 year / 1,026 children) and Asahi University (3 years / 181 children)
Results:
• Nano medical hydroxyapatite significantly lowered the incidence of new caries in previously and newly erupted teeth.
• In the 3-year study, the reduction was as high as 36 % to 56 %.
1993:
The Japanese government approves Sangi's proprietary medical hydroxyapatite as an active anti-caries agent.

2003:
Sangi increases the enamel-restorative capabilities of medical hydroxyapatite by reducing its nanoparticles from 100 to 50 nanometers (1 nanometer = 1 millionth of a millimeter), making it more effective at penetrating below the surface of the enamel.
2013:
• 100 million medical hydroxyapatite toothpastes sold by Sangi over the last 35 years.
• Nano medical hydroxyapatite is now the gold standard in Japan to fight cavities.
2015:
X-PUR Remin is granted the Health Canada indication of a fluoride free toothpaste that helps reduce caries.
